Antiscalant
Avista Technologies Global
What is Antiscalant ?
- Antiscalant is a pretreatment water additive for reverse osmosis system that is highly effective in preventing the membranes from scaling.
- Before the feed water enters the reverse osmosis membrane, an antiscalant is injected into the water and sent the through the system.
- Antiscalants are a family of chemicals designed to inhibit the formation and precipitation of crystallized mineral salts that form scale.
- Most antiscalants are proprietary organic man-made polymers (e.g., polyacrylic acids, carboxylic acids, polymaleic acids, organophosphates, polyphosphates, phosphonates, anionic polymers, etc.).
- The molecular weight of these polymers can range from 2000 to 10,000 Da.
Why use Antiscalant ?
A scale is soluble material in water which precipitates, becomes insoluble, and deposits on a surface.
Various factors like temperature, pH, alkalinity, over saturation, or a change in water chemistry can affect the development of scale.
The most common mineral scalants of concern are:
1. Calcium carbonate (CaCO3)
2. Calcium sulfate (CaSO4)
3. Strontium sulfate (SrSO4)
4. Barium sulfate (BaSO4)
Less common mineral scalants are:
1. Calcium phosphate [Ca3(PO4)2]
2. Calcium fluoride (CaF2)
Chemistry Behind Antiscalant
Chemistry behind Antiscalant
- Antiscalant is a pre-treatment water additive for reverse osmosis system that is highly effective in preventing the membranes from scaling.
- Before the feed water enters the reverse osmosis membrane, an antiscalant is injected into the water and sent the through the system.
- The chemicals creates a time delay between the bicarbonate and the calcium magnesium.
- The delay allows the water to pass through the membrane before any chemical reaction, in which scale can form, occurs.
- This results is scale not forming as the water is being purified by the RO.
- Maintaining proper dosing levels of an antiscalant/dispersant is important.
- Under dosing can cause scaling or fouling.
- Overdosing can cause a deposition of the antiscalant/dispersant onto the membrane, creating a fouling problem.
Over the years, the RO industry has developed methods of scale prevention.
These methods fall into three categories:
- Acidification
- Ion exchange softening
- Scale inhibitor addition
Acidification Chemistry
Acidification
- Acidification shifts bicarbonate and carbonate alkalinity needed to produce calcium carbonate scale as illustrated by the following equations
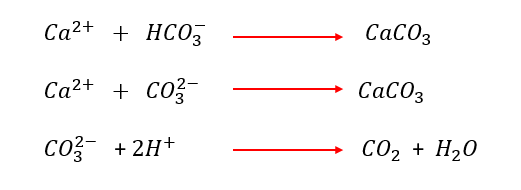
- But is not effective against the sulfate scales of calcium, barium, and strontium.
- Additional disadvantages of acidification include corrosivity of the acid, the cost of storage tanks, and the monitoring equipment required for proper acid dosing.
- Acidification also lowers permeate pH, requiring post-treatment pH adjustment or degassers to remove excess carbon dioxide.
Ion Exchange Chemistry
Ion Exchange
- Ion exchange softening utilizes the sodium form of cation resin.
- In this process, hardness ions in the water exchange for sodium ions contained in the resin.
- The following equations illustrate these softening reactions. NaZ represents cation resin in the sodium form.

- There are anecdotal accounts of ion exchange softening reducing colloidal and particulate fouling of RO systems by increasing the electrostatic charge on the colloidal and particulate solids.
- However, to date there is no convincing evidence to support this claim.
- When compared to either acid or scale inhibitor addition, the main disadvantage of ion exchange softening is cost.
Scale Inhibitor Chemistry
Scale Inhibitor Addition
Scale inhibitors are surface-active substances that prevent precipitation of sparingly soluble salts by three different mechanisms.
- Threshold inhibition
- Crystal modification
- Dispersion
Threshold Inhibition chemistry
- Threshold inhibition is the delaying of crystal formation by scale inhibitors at very low concentrations.
- Physical chemists call this delay an induction period. Prior to crystallization, crystal nuclei begin to assemble on a sub-microscopic scale.
- Scale inhibitors act as structure breakers by associating with cations present in the assembling nuclei.
- Eventually crystal nuclei greatly outnumber inhibitor molecules, and crystallization of the sparingly soluble salt begins.
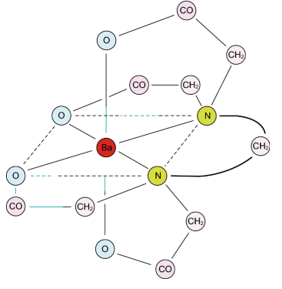
Crystal Modification Chemistry
- Scale inhibitors incorporate into growing crystal structures and distort their shape.
- Crystals thus modified are slower to grow on membrane surfaces.
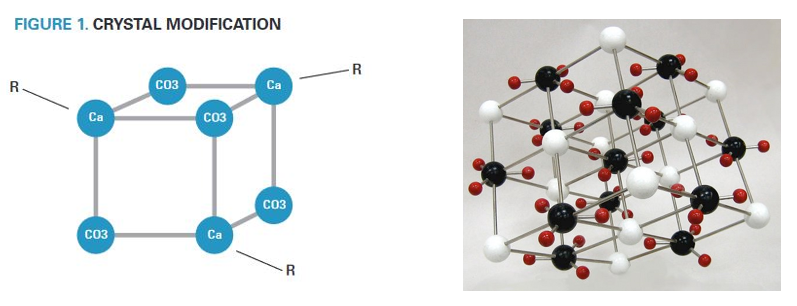
- Figure 1 illustrates the incorporation of inhibitor molecules into the crystal structure by association of the crystal cations with negative functional groups present on the inhibitor.
- R represents the inhibitor molecule.
The greater negative electrostatic charge coupled with steric hindrance, created by the adsorbed inhibitor, increases the repulsion between the colloids and particulates, again delaying crystal growth on membrane surfaces.
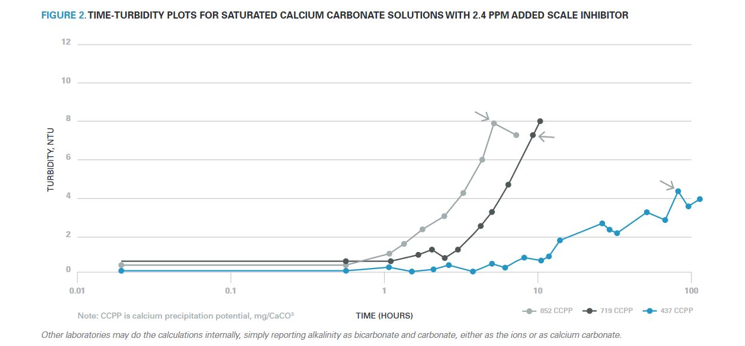
Figure 2 illustrates the delay in crystallization caused by the addition of 2.4 mg/l of a neat scale inhibitor to supersaturated solutions of calcium carbonate.
Sudden increases in turbidity signal the onset of crystallization
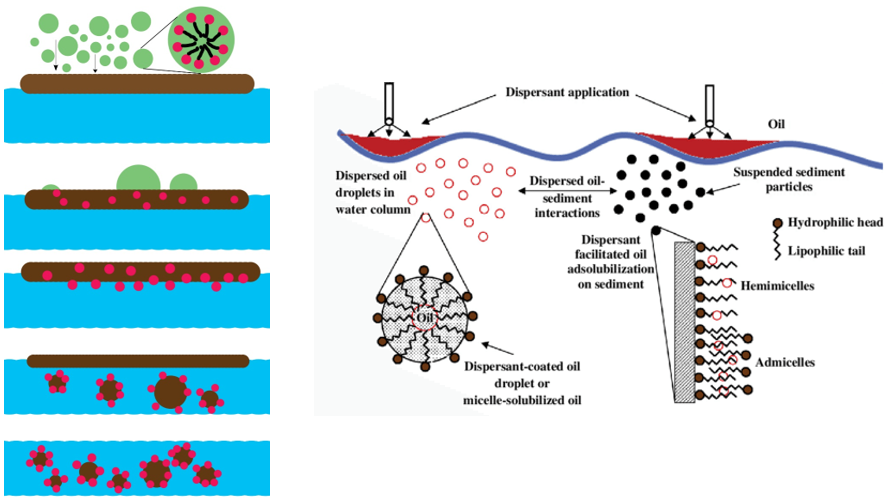
Dispersant Chemistry
- A dispersant or a dispersing agent is a substance, typically a surfactant, that is added to a suspension of solid or liquid particles in a liquid to improve the separation of the particles and to prevent their settling or clumping.
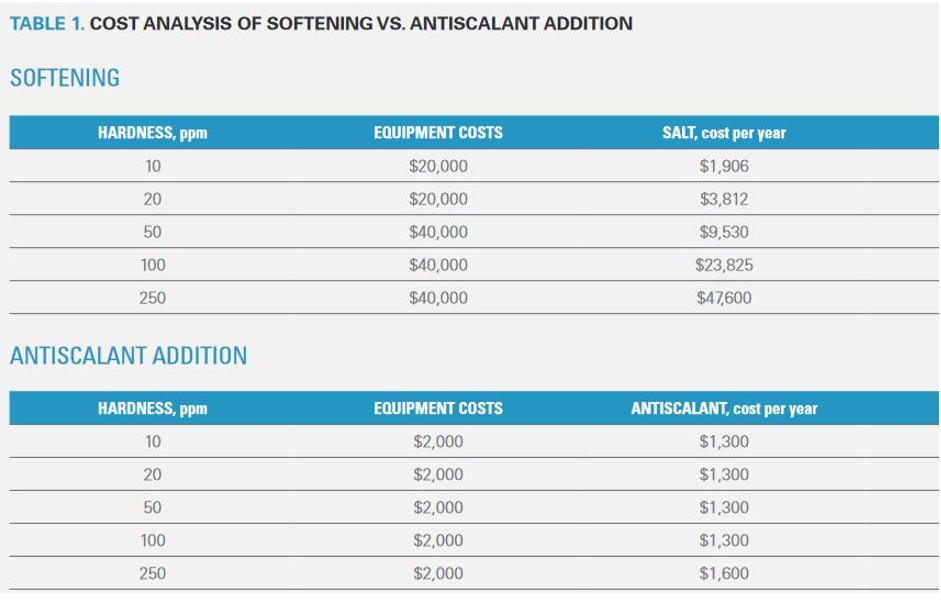
Cost Effectiveness
Vitec™ 5100
- A dispersant or a dispersing agent is a substance, typically a surfactant, that is added to a suspension of solid or liquid particles in a liquid to improve the separation of the particles and to prevent their settling or clumping.

Creative Chemistry. Smart Solutions
PRODUCT INFORMATION
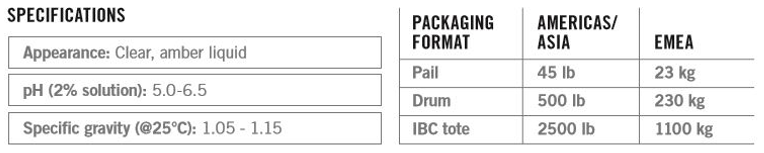
Packaging and Storage
Standard regional pack sizes are listed below. Information on drumless or bulk tanker delivery is available on request.
- Vitec 5100® is a broad spectrum, liquid antiscalant and dispersant that is compatible with organic coagulants and effective at low dose rates.
- This multicomponent formulation is targeted at preventing scale precipitation in larger systems and is particularly cost effective in seawater and municipal reverse osmosis (RO) applications.
- Vitec 5100 is compatible with our line of RoQuest® coagulants and certified by NSF International under NSF/ANSI Standard 60 for use in systems producing drinking water.
- This highly concentrated antiscalant can be used at very low doses and is good for wastewater and well water with high silica.
- Powerful against these and other types of scale:
Calcium carbonate (CaCO3)
Calcium phosphate (CaPO4)
Silica (SiO2)
Calcium fluoride (CaF2)
Calcium sulfate (CaSO4)
Barium sulfate (BaSO4)
Strontium sulfate (SrSO4)
- Good for wastewater and ground water containing elevated calcium phosphate or silica concentrations.
- Dispersants in this product break up clays and particulates.
- Highly effective at low dose rates in a wide variety of feed waters and pH ranges.
- Controls scale and inorganic fouling.
- Compatible with membranes from all manufacturers and Avista™ coagulants.
Instruction for Use
Injecting
Best results are achieved when Vitec 5100 is injected upstream of the membrane system and, where possible, of cartridge filters.
Dosing
A typical Vitec 5100 dose ranges from 2-5 mg/l.
A site-specific dose can be determined using the
Avista Advisor software program.
Dilution
Vitec 5100 is formulated to be injected neat.
However, if dilution is required, use deionized water or RO permeate.
If neither of these is available, softened water may be substituted.
The dilution for Vitec 5100 should not result in a solution strength of less than 10%.
This guideline will protect the effectiveness of the internal bacteriostatic, which inhibits bacterial growth in the product packaging and feed tank.
Calculation
Typically, a scale inhibitor/dispersant dosage rate of 2 - 5 milligrams per liter (mg/L) is adequate to prevent scaling.
Example :
Feed Flow: 100 m3/h
Specified Dose: 3 mg/l neat solution (provide by chemical supplier)
Anti-Scalant Specific gravity : 1.15
Antiscalant Gravity : 1150 kg/m3
100 m3/h x 24 h/day = 2400 m3/day
3 mg/l (ppm) x 2400 x 1000 = 7,200,000 mg/day
7,200,000 mg/day / 1150 kg/m3 = 6.261 l/day per train
6.261 l/day x 1000 ml = 6261 ml/day
6261 ml/day / 1440 min/day = 4.3 ml/min
Therefore, your dosage should be set at the dosing pump to 4.3 ml/min for this particular example.
Health effect of Antiscalant
- Antiscalants are non-toxic, or they would not be approved by NSF for potable water applications.
- Both phosphonate and polymer based antiscalants are biodegradable over time.
- Ultraviolet light increases the rate of degradation as the chemical bonds are broken, and certain types of bacteria produce enzymes that cleave the bonds.
- Recently some “green” antiscalants have been introduced into the market, with the claim that they biodegrade at a faster rate.
Storage
- Store containers in a cool, dry location, away from direct sunlight, sources of intense heat, or where freezing is possible.
- Store away from incompatible materials.
- Material should be stored in secondary containers, or in a diked area, as appropriate.
- Storage and use areas should be covered with impervious materials .
- Keep container tightly closed when not in use.
- If appropriate, post warning signs in storage and use areas.
- Inspect all incoming containers before storage, to ensure containers are properly labelled and riot damaged.
Handling
- All employees who handle this material should be trained to handle it safely.
- Open containers carefully and on a stable surface.
- Empty containers may contain residual liquid; therefore, empty containers should be handled with care.
- As with all che1nicals, avoid getting this product on you or in you.
- Wash thoroughly after handling this product.
- Do not eat or drink while handling this material.
- A void generating mists and sprays of this product Remove contaminated clothing immediately.
